3.4.2.9 Sintered
fused silica
Silicon oxide ceramics (SiO2)
(also known as fused silica ceramics or quarzware5)
are sintered from (amorphous) silicon dioxide powders. The
goal is to obtain the typical properties of silicon dioxide,
with its thermal expansion coefficient very close to zero,
in ceramic components where those properties can be exploited.
If the process is appropriately controlled, it is possible
to retain the (amorphous) silicon dioxide phase in the sintered
ceramic. The result is a ceramic with relatively low strength
compared to the high strength oxide and non-oxide ceramics,
but which has an extremely high thermal shock resistance as
a direct result of the extremely low thermal expansion of
the amorphous silicon dioxide phase.
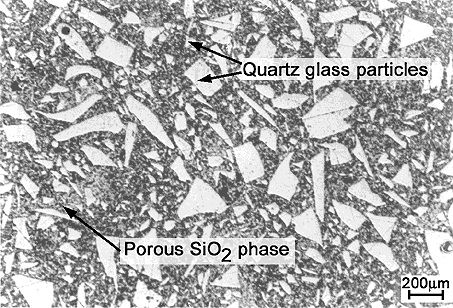
Figure 29: Microstructure of sintered fused
silica
The maximum application temperature of 1,050°
C should not be exceeded. Correspondingly, the application
of these ceramics lies in areas where extreme thermal shocks
are experienced and in the
5)
The term ‘Quartzware’ originates from the manufacture
of quartz glass. Due to the wide variety of methods used to
manufacture more or less pure SiO2 glasses, they are grouped
nowadays from the scientific point of view under the general
heading of silica glass. Chemically, transparent silica (quartz)
glass, which is manufactured from a quartz sand or rock crystal,
is practically identical to non-transparent quartzware ceramics.
The primary difference between silica glass and quartzware
is therefore just the different transparencies. From a scientific
point of view, the term ceramic quartzware is wrong, since
it does not contain crystalline phase SiO2, but just particles
of quartz glass.
|
|

